The power of ozone, its relationship with the development of speech and the area of storage. Ozone control in water Ozone control in water
OZONE (O 3) is an allotropic modification of acidity, the molecule is folded from three atoms into color and possibly from all three aggregate mills. Molecule ozone has a structure in the form of a ryon-femoral tricycle with a top of 127 o. However, the closed tricycle does not establish itself, but the molecule of lantsyuga cannabis has 3 atoms in the form of 0.224 nm. Depending on the molecular structure, the dipole moment of the warehouse is 0.55 debye. In the electronic structure, ozone molecules are numbered 18 electrons, which constitute a mesomeric-stable system, which is used in other near-Cordon countries. Prikordonny ion structures represent the dipole character of the ozone molecule and explain its specific reaction behavior in terms of sour, which is a radical with two unpaired electrons. The ozone molecule is composed of three atoms. Chemical formula for gas-O 3 Reaction to ozone: 3O 2 + 68 kcal / mol (285 kJ / mol) ⇄ 2O 3 Molecular weight to ozone - 48 At room temperature ozone is a nonbarvable gas with a characteristic odor. The smell of ozone is perceptible at a concentration of 10 -7 M. In a native country, ozone is a dark blue color with a melting temperature of -192.50 C. Solid ozone is a crystal of a black color with a temperature of -111.9 degrees C. At temperatures 0 gr. і 1 atm. = 101.3 kPa, the ozone concentration becomes 2.143 g / l. In a gas-like mill, ozone is diamagnetic and blows out of a magnetic field, in a gas-like mill, it is weakly paramagnetic, so that it is volodyne. magnetic field and be drawn into the magnetic field.
Chemistry for ozone
The ozone molecule is non-volatile, and at sufficient concentrations in the heat with normal minds, it is transformed into a diatomic acid with the appearance of heat. Adjusting the temperature and reducing the pressure to increase the efficiency of ozone distribution. Contact ozone with small quantities organic speeches, Deyakikh metals or oxides will quickly accelerate the transformation. Chemically active ozone is even more high, even strong oxidizing. All metals were oxidized, and they were all metal (behind a vinatka of gold, platinum and iridium) and a lot of non-metals. The product of the reaction is mainly kissen. Ozone can be found in water more colorfully, less pink, no tricky solution, moreover, the speed of its distribution in the range of 5-8 times in food, not in the gas phase, not in the gas phase (Rozumovskiy S.D., 1990). The price is evidently not due to the specificity of the condensed phase, but due to the reactions with the houses and the ion of hydroxyl; The relationship between ozone and sodium chloride is consistent with the Henry's law. With the increase in NaCl concentration in the water release, the ozone release rate decreases (Tarunina V.N. і spіvavt., 1983). Ozone is even less highly competitive up to an electron (1.9 eV), which is a strong oxidizing agent, which is superior to fluorine (Rozumovskiy S.D., 1990).Biological power to ozone and its infusion on the human body
Visoka oxidizing building and those who are in the bagatokh chemistry reactions, which push for the participation of ozone, pretend to be sour radicals, curb the whole gas in the edge is not safe for people. Yak gas-like ozone injected onto lyudin:- Dratu і poshkozhu fabrics of organіv dikhannya;
- Pouring on cholesterol in the blood of a human being, perceiving neural forms, leading to atherosclerosis;
- Dovge znakhozhennya in the middle of the increased concentration of ozone can be the reason for the cholovic bezpliddya.
- The maximum one-time boundary permissible concentration (GDK m.r.) in atmospheric food population m_sc 0.16 mg / m 3
- Medium-like boundary permissible concentration (GDK d.w.) - 0.03 mg / m 3
- The boundary permissible concentration (HDC) in the second working zone is 0.1 mg / m 3 (at the same time, the time for human scent is close to 0.01 mg / m 3).
High and low power to ozone
Ozone is present in two spheres of the atmosphere. Tropospheric or ground-level ozone, which is located in the closest sphere of the atmosphere to the surface of the Earth, is not safe in the troposphere. Win shkidliviy for people, and for their living organisms. Wine is shedding teeth on the trees, to grow the silky-diversified cultures. In addition, tropospheric ozone is one of the leading "Ingredіntіv" of Moscow I can. At the same hour, stratospheric ozone is even cinnamon. Ruynuvannya set by him ozone ball (ozone screen) to produce before the flow of ultraviolet viprominuvannya to the earth's surface to grow. Through the growth of a number of illnesses for cancer of the shkiri (including the most notable type of melanoma), cataract infections. Injection of hard ultraviolet will weaken immunity. Overwhelming UV exposure can also be a problem for Silskoy government So, how can you make your crops so sensitive to ultraviolet. At the same hour, the memory slid, scho ozone is an off-gas, and at rivn earthly surface win є we will be hard-hitting. Influenced through an intense sleepy vipromynuvannya and specs in every turn to pretend to be especially rich in luscious ozone.Vzaєmodiya ozone and sour one by one. Details and details.
Ozone is an allotropic form of acid. Allotropy is the manifestation of one and the same chemical element in two or more simple words. In this case, ozone (O3) and oxygen (O 2) are approved a cheesy element O. Otrimannya to ozone from acidity Yak, as a rule, by way of discarding ozone, molecular oxygen (O 2) appears, and the process itself is described by the equivalents 3O 2 → 2O 3. The reaction is endothermic and easily reversible. For substitution of equilibrium in the whole product (ozone), come in. One of the ways to eliminate ozone is to use an arc discharge. Thermal dissociation of molecules with increasing temperature. So, at T = 3000K - zm_st of atomic sourness becomes ~ 10%. The temperature in a few thousand degrees can be adjusted for an additional arc discharge. However, at high temperatures, ozone develops faster than molecular sourness. It’s possible to get rid of it, it’s possible to change it, heat the gas, and then quickly cool it down. Ozone in this form is an intermediate product during the transition from sum O 2 + O to molecular acid. The maximum concentration of O 3, yaku can be trimmed with this method of virobnitstva, reachє 1%. Quite enough for great industrial purposes. Oxidation power to ozone Ozone is a strained oxidizing agent, which is more reactive in porous acidic acid. Oxidized may be all metals and non-metals according to the statement of acidity: 2 Cu 2+ (aq) + 2 H 3 O + (aq) + O 3 (g) → 2 Cu 3+ (aq) + 3 H 2 O (1) + O 2 (g) Ozone can play a role in the reactions of the furnace, the temperature of the furnace at the whole temperature, lower at the temperature in the atmosphere of a diatomic acid: 3 C 4 N 2 + 4 O 3 → 12 CO + 3 N 2 The standard potential for ozone is 2.07 V, To this, the ozone molecule does not change and transforms into heat. At low concentrations, ozone develops more often, with high concentrations - with vibuch, which means that the molecule lacks excess energy. Heating and contacting ozone with small particles of organic fluids (hydroxide, peroxide, metal of low valence, and oxides) quickly accelerates the transformation. Navpaks, the presence of small amounts of nitric acid and stabilizes ozone, and in vessels from a storey and some plastics or pure metals, ozone at -78 0 C. is practically unfolded. Competitiveness of ozone to the electronic door 2 ev. So strong is the spore of the volodin only fluorine and that oxide. Ozone oxidized all metals (behind a vignette of gold and platinum), as well as a large number of other elements. Chlorine takes care of the part in the reactions with ozone and hypochlorite ОCL. Reactions to ozone with atomic water є dzherel for the establishment of hydroxyl radicals. Ozone has a maximum moisture content in the UV region at an increase of 253.7 nm with a molar coefficient of extinction: E = 2,900 At the given UV-photometric value of the ozone concentration at the same time with the iodine-metric titration of the adopted standards. Kisen, on the basis of ozone, does not enter into the reaction with KI.Ozone and water stability
The liquidity of ozone distribution in the range of 5-8 times in food, lower in the gas phase. Ozone breakdown in water is 10 times higher, lower acidity. According to the data of the previous authors, the value of the efficiency of ozone in water varies from 0.49 to 0.64 ml of ozone / ml of water. In ideal thermodynamic minds, it is necessary to adhere to the law of Henry, so that the concentration of the gas that is broken down is proportional to its partial vice. C S = B × d × Рi de: С S - the concentration of the broth in water; d - mass to ozone; Pi - partial grip of ozone; B - calculation efficiency; Determination of the law of Genri for ozone as a metastable gas is cleverly. The drop in ozone in the gas phase lies in a partial vice. At the water center, there are some processes that go beyond the area of the law of Genri. The Gibs-Dukem-Margulesdu law will replace the new one in the ideal minds. Practically, it is accepted to rotate the difference in ozone in water through the reduction of ozone concentration in the middle soil to the concentration of ozone in the gas phase: With the same sinks in tap water, ozone concentration becomes 13 mg / l, in double-distilled water - 20 mg / l. The reason for this is a significant drop in ozone through the winter houses near the drinking water.Decrease in ozone і period overіvrozpadu (t 1/2)
At the water center, ozone falls strongly in the water quality, temperature and pH of the middle. Increasing the pH of the middle soil will accelerate the ozone drop and lower the ozone concentration in water. Similar processes occur at an adjustable temperature. The period for ozone fall in bidistilled water becomes 10 years, in demineralized water - 80 quilines; in distilled water - 120 quilins. Seemingly, the distribution of ozone in water є by the folding process of reactions of radicals: The maximum amount of ozone in a water eye is prevented from being consumed by a stretch of 8-15 minutes. After 1 year, only a few radicals will appear. Among them, we have found the hydroxyl radical (OH ') (Staehelin G., 1985), and it is necessary to take up the respect for vikorystanny ozone water for therapeutic purposes. Oscillations in clinical practice to know the amount of ozonized water and ozonovane physiological changes, we have carried out an assessment of the amount of ozonovane in the amount of ozone in the concentration, as it is in the prevailing medical conditions. The main methods of analysis were the iodometric titration and intensity of chemiluminescence from the microcircuitry attached to the biochemiluminometer BKhL-06 (Nizhniy Novgorod) (Kontorshchikova K.N., Peretyaginov S.P., 1995). The phenomenon of chemiluminescence is associated with the reactions of recombination of vilny radicals, which are established when ozone is placed in water. When sampling 500 ml of bi-distilled water and bubbling ozone-sour gas sum with ozone concentration in the boundaries of 1000-1500 mcg / l and a quick flow of gas 1 l / h in a stretch of 20 chilines, chemiluminescence vyvii 160 Moreover, in double-distilled water, the intensity of light is purely, but not in distilled water. Ozone tolerance in NaCl ratios complies with Henry's law, in order to decrease due to the increase in salt concentration. Physiological breakdowns were doused with ozone at a concentration of 400, 800 and 1000 μg / L with a stretch of 15 quilins. Global light intensity (in mv) increased due to growing ozone concentration. The triviality of the wedding is to become 20 khvili. The price is explained by the large-scale recombination of strong radicals and the results of extinguishing the light for the manifestation of the physiological development of the houses. Unimportant to high oxidation potential, ozone is highly selective, as it is encumbered with polar budic molecules. Mittely react with ozone on a half-hour basis, so that you can replace a subtle sound (-C = C-). As a result, sensitive to ozone є unsaturated fatty acids, aromatic amino acids and peptides, perch for all SH-groups. It follows from the data of Krege (1953) (cited by Vieban R. 1994), the first product of the interaction of ozone molecules with biological substrates - 1-3 dipolar molecules. The reaction is the main one in the interaction of ozone with organic substrates at pH< 7,4.
Озонолиз проходит в доли секунды. В растворах скорость этой реакции равна 105 г/моль·с. В первом акте реакции образуется пи-комплекс олефинов с озоном. Он относительно стабилен при температуре 140 0 С и затем превращается в первичный озонид (молозонид) 1,2,3-триоксалан. Другое возможное направление реакции — образование эпоксидных соединений.
Первичный озонид нестабилен и распадается с образованием карбоксильного соединения и карбонилоксида. В результате взаимодействия карбонилоксида с карбонильным соединением образуется биполярный ион, который затем превращается во вторичный озонид 1,2,3 — триоксалан. Последний при восстановлении распадается с образованием смеси 2-х карбонильных соединений, с дальнейшим образованием пероксида (I) и озонида (II).
The maximum amount of ozone in a water eye is prevented from being consumed by a stretch of 8-15 minutes. After 1 year, only a few radicals will appear. Among them, we have found the hydroxyl radical (OH ') (Staehelin G., 1985), and it is necessary to take up the respect for vikorystanny ozone water for therapeutic purposes. Oscillations in clinical practice to know the amount of ozonized water and ozonovane physiological changes, we have carried out an assessment of the amount of ozonovane in the amount of ozone in the concentration, as it is in the prevailing medical conditions. The main methods of analysis were the iodometric titration and intensity of chemiluminescence from the microcircuitry attached to the biochemiluminometer BKhL-06 (Nizhniy Novgorod) (Kontorshchikova K.N., Peretyaginov S.P., 1995). The phenomenon of chemiluminescence is associated with the reactions of recombination of vilny radicals, which are established when ozone is placed in water. When sampling 500 ml of bi-distilled water and bubbling ozone-sour gas sum with ozone concentration in the boundaries of 1000-1500 mcg / l and a quick flow of gas 1 l / h in a stretch of 20 chilines, chemiluminescence vyvii 160 Moreover, in double-distilled water, the intensity of light is purely, but not in distilled water. Ozone tolerance in NaCl ratios complies with Henry's law, in order to decrease due to the increase in salt concentration. Physiological breakdowns were doused with ozone at a concentration of 400, 800 and 1000 μg / L with a stretch of 15 quilins. Global light intensity (in mv) increased due to growing ozone concentration. The triviality of the wedding is to become 20 khvili. The price is explained by the large-scale recombination of strong radicals and the results of extinguishing the light for the manifestation of the physiological development of the houses. Unimportant to high oxidation potential, ozone is highly selective, as it is encumbered with polar budic molecules. Mittely react with ozone on a half-hour basis, so that you can replace a subtle sound (-C = C-). As a result, sensitive to ozone є unsaturated fatty acids, aromatic amino acids and peptides, perch for all SH-groups. It follows from the data of Krege (1953) (cited by Vieban R. 1994), the first product of the interaction of ozone molecules with biological substrates - 1-3 dipolar molecules. The reaction is the main one in the interaction of ozone with organic substrates at pH< 7,4.
Озонолиз проходит в доли секунды. В растворах скорость этой реакции равна 105 г/моль·с. В первом акте реакции образуется пи-комплекс олефинов с озоном. Он относительно стабилен при температуре 140 0 С и затем превращается в первичный озонид (молозонид) 1,2,3-триоксалан. Другое возможное направление реакции — образование эпоксидных соединений.
Первичный озонид нестабилен и распадается с образованием карбоксильного соединения и карбонилоксида. В результате взаимодействия карбонилоксида с карбонильным соединением образуется биполярный ион, который затем превращается во вторичный озонид 1,2,3 — триоксалан. Последний при восстановлении распадается с образованием смеси 2-х карбонильных соединений, с дальнейшим образованием пероксида (I) и озонида (II).
 Ozonation of aromatic spoluks against the statements of polymeric ozonides. Applying ozone will deplete aromatic energy in the nucleus and vitrate energy, which is why the efficiency of ozone homologues is the main reason for the energy production. Ozonuvannya of dried in carbohydrates is tied to the mechanism of vprovadzhennya. Ozonuvannya siro and nitrogen mixed organic spoluk in the following way:
Ozonation of aromatic spoluks against the statements of polymeric ozonides. Applying ozone will deplete aromatic energy in the nucleus and vitrate energy, which is why the efficiency of ozone homologues is the main reason for the energy production. Ozonuvannya of dried in carbohydrates is tied to the mechanism of vprovadzhennya. Ozonuvannya siro and nitrogen mixed organic spoluk in the following way:  Ozonіdi zazvychay nasty razchinyayutsya at the water, alas, in organic razchinniki. When heated, some transition metals fall to radicals. The number of ozonides in organic form is the iodine number. Iodine number - the mass of iodine in grams, which was added to 100 g of organic speech. For fatty acids, the iodine number should not be 100-400, for solid fats 35-85, for hard fats - 150-200. Forward ozone, as an antiseptic one, was used by A. Wolff in 1915 for the first time. The coming fate has been step-by-step accumulating information about the success of ozone consumption in cases of illicit diseases. However, for a trivial hour, the vikorists were deprived of the ozone therapy method, connected with direct contacts to ozone from the ringing surfaces and the thin empty spaces. I am interested in ozone therapy, having seen in the world the accumulation of data about the biological effect of ozone on the body, and you will have a chance to hear about the success of ozone testing in case of catching a whole series of problems. History medical supply ozone has been repaired since the 19th century. Pioneers of the classic ozone supply in Western America and Europe, zokrem, C. J. Kenworthy, B. Lust, I. Aberhart, E. Payer, E. A. Fisch, N. N. Wolff and іnshі. Russia has very little about the consumption of ozone. Tilki in 60-70 years in the last years of literature from the widespread use of the widespread method of developing ozone therapy and on the consumption of ozone in the population of young children in the region, and in the 80s in the history of our The bases for fundamental development of ozone therapy technologies are quite abundant in the form of robots to the Institute of Chemistry Physics of the Academy of Medical Sciences of the USSR. The book "Ozone and Yogo Reactions with Organic Speeches" (SD Rozumovskiy, G. Ye. Zaikov, Moscow, 1974) became a reference point for the development of mechanisms in the economic development of ozone in bagatians. The International Ozone Association (IOA) has a wide range of activities, which has held 20 international congresses, and since 1991, our doctors and sciences have taken part in robotic congresses. We call in a new way to look at the problems of applied ozone treatment, and in medicine itself. In the therapeutic range of concentrations and doses of ozone, the power of a push-pull bioregulator, which is quite abundant in terms of the methods of traditional medicine, and most often in the form of monotherapy. Zasosuvannya medicinal ozone represents a clear novelty of topical problems in the development of health problems. Ozone therapy technologies are used in surgery, obstetrics and gynecology, dentistry, neurology, in case of therapeutic pathology, infectious ailments, dermatology and venereal ailments and a number of others. Ozone therapy is characterized by simplicity, high efficiency, good tolerance, practical visibility of side effects, it is economically viable. Likuvalny power to ozone in cases of disease unique building vlivati on organizm. Ozone in therapeutic doses for immunomodulatory, anti-pyretic, bactericidal, anti-skin, fungicidal, cytostatic, anti-stress and analgesic. Yogo zdatnist actively correcting damage to acid homeostasis to the body has great prospects for intravenous medicine. A wide range of methodical possibilities allows, due to the great efficiency of vikoristovuvuvat of the ozone for muscle and systemic therapy. In the last ten years, methods have come to the fore, related to parenteral (internal, internal, internal, internal) introduced therapeutic doses of ozone, active systemic effects of Kisnevo-ozone gaseous sum with high (4000 - 8000 mcg / l) concentrations in low ozone is effective in case of severely inflamed, foul wounds, gangrene, decubitus ulcers, diagnoses, fungal infections of the school, etc. Ozone in high concentrations can also be used as blood-backed ones. Low concentration of ozone stimulates reparation, energy efficiency and ignition. In some colitis, proctitis, norits, and in a number of other intestinal problems, the rectal administration of acid-ozone gas sums is rectal. Ozone, according to the physiological breakdown, successfully stagnates during peritonitis for the sanitation of black waste, and ozonized water is distilled in a slit surgery and in. For the internal introduction of ozone vikoristovuyutsya, razchinny at physiological razchini abo in the blood of a sick person. The pioneers of the European school have been following the postulate about those who by means of ozone therapyє: "Stimulation and reactivation of acid metabolism without disrupting the oxidative-active systems", - this means that when the doses are taken per session or the course, the ozone therapy infusion can be used in between . Riling, R. FIBA 1996 in the book. Practice of ozone therapy). In foreign medical practice for parenteral administration of ozone vikoristovuyutsya, in the main, large and small autohemotherapy. During the great autohemotherapy, the patient's roof was taken from the patient to get rid of the oxygen-ozone gas mixture, and immediately drip back into the vein of the same patient. With little autohemotherapy, the ozonated roof is introduced internally. A therapeutic dose of ozone in the whole range is shown for a mixture of gas and ozone concentration in a new one.
Ozonіdi zazvychay nasty razchinyayutsya at the water, alas, in organic razchinniki. When heated, some transition metals fall to radicals. The number of ozonides in organic form is the iodine number. Iodine number - the mass of iodine in grams, which was added to 100 g of organic speech. For fatty acids, the iodine number should not be 100-400, for solid fats 35-85, for hard fats - 150-200. Forward ozone, as an antiseptic one, was used by A. Wolff in 1915 for the first time. The coming fate has been step-by-step accumulating information about the success of ozone consumption in cases of illicit diseases. However, for a trivial hour, the vikorists were deprived of the ozone therapy method, connected with direct contacts to ozone from the ringing surfaces and the thin empty spaces. I am interested in ozone therapy, having seen in the world the accumulation of data about the biological effect of ozone on the body, and you will have a chance to hear about the success of ozone testing in case of catching a whole series of problems. History medical supply ozone has been repaired since the 19th century. Pioneers of the classic ozone supply in Western America and Europe, zokrem, C. J. Kenworthy, B. Lust, I. Aberhart, E. Payer, E. A. Fisch, N. N. Wolff and іnshі. Russia has very little about the consumption of ozone. Tilki in 60-70 years in the last years of literature from the widespread use of the widespread method of developing ozone therapy and on the consumption of ozone in the population of young children in the region, and in the 80s in the history of our The bases for fundamental development of ozone therapy technologies are quite abundant in the form of robots to the Institute of Chemistry Physics of the Academy of Medical Sciences of the USSR. The book "Ozone and Yogo Reactions with Organic Speeches" (SD Rozumovskiy, G. Ye. Zaikov, Moscow, 1974) became a reference point for the development of mechanisms in the economic development of ozone in bagatians. The International Ozone Association (IOA) has a wide range of activities, which has held 20 international congresses, and since 1991, our doctors and sciences have taken part in robotic congresses. We call in a new way to look at the problems of applied ozone treatment, and in medicine itself. In the therapeutic range of concentrations and doses of ozone, the power of a push-pull bioregulator, which is quite abundant in terms of the methods of traditional medicine, and most often in the form of monotherapy. Zasosuvannya medicinal ozone represents a clear novelty of topical problems in the development of health problems. Ozone therapy technologies are used in surgery, obstetrics and gynecology, dentistry, neurology, in case of therapeutic pathology, infectious ailments, dermatology and venereal ailments and a number of others. Ozone therapy is characterized by simplicity, high efficiency, good tolerance, practical visibility of side effects, it is economically viable. Likuvalny power to ozone in cases of disease unique building vlivati on organizm. Ozone in therapeutic doses for immunomodulatory, anti-pyretic, bactericidal, anti-skin, fungicidal, cytostatic, anti-stress and analgesic. Yogo zdatnist actively correcting damage to acid homeostasis to the body has great prospects for intravenous medicine. A wide range of methodical possibilities allows, due to the great efficiency of vikoristovuvuvat of the ozone for muscle and systemic therapy. In the last ten years, methods have come to the fore, related to parenteral (internal, internal, internal, internal) introduced therapeutic doses of ozone, active systemic effects of Kisnevo-ozone gaseous sum with high (4000 - 8000 mcg / l) concentrations in low ozone is effective in case of severely inflamed, foul wounds, gangrene, decubitus ulcers, diagnoses, fungal infections of the school, etc. Ozone in high concentrations can also be used as blood-backed ones. Low concentration of ozone stimulates reparation, energy efficiency and ignition. In some colitis, proctitis, norits, and in a number of other intestinal problems, the rectal administration of acid-ozone gas sums is rectal. Ozone, according to the physiological breakdown, successfully stagnates during peritonitis for the sanitation of black waste, and ozonized water is distilled in a slit surgery and in. For the internal introduction of ozone vikoristovuyutsya, razchinny at physiological razchini abo in the blood of a sick person. The pioneers of the European school have been following the postulate about those who by means of ozone therapyє: "Stimulation and reactivation of acid metabolism without disrupting the oxidative-active systems", - this means that when the doses are taken per session or the course, the ozone therapy infusion can be used in between . Riling, R. FIBA 1996 in the book. Practice of ozone therapy). In foreign medical practice for parenteral administration of ozone vikoristovuyutsya, in the main, large and small autohemotherapy. During the great autohemotherapy, the patient's roof was taken from the patient to get rid of the oxygen-ozone gas mixture, and immediately drip back into the vein of the same patient. With little autohemotherapy, the ozonated roof is introduced internally. A therapeutic dose of ozone in the whole range is shown for a mixture of gas and ozone concentration in a new one. The scientific achievements of the vicarians began to be regularly updated at international congresses and symposiums
- 1991 - Cuba, Havana,
- 1993 - USA San Francisco,
- 1995 - FranziaLill,
- 1997 rock - Japan, Kyoto,
- 1998 - Austria, Salzburg,
- 1999 r. - Nimechchina, Baden-Baden,
- 2001 - England, London,
- 2005 - France, Strasbourg,
- 2009 - Japan, Kyoto,
- 2010 rock - Spain, Madrid
- 2011 Turkey (Istanbul), France (Paris), Mexico (Cancun)
- 2012r. - Spain Madrid
All-Russian Scientific and Practical Conferences with International Ozone Therapy
I - "OZONE IN BIOLOGIS TA MEDICINI" - 1992 r., N.Novgorod II - "OZONE IN BIOLOGIS TA MEDICINI" - 1995 r., N.Novgorod III - "OZONE I EFFECTIVE THERAPY METHODS" - 1998 r., N.Novgorod IV - "OZONE I EFFECTIVE THERAPY METHODS" - 2000 RUR., N.Novgorod V - "OZONE IN BIOLOGIS TA MEDICIN" - 2003 r., N.Novgorod VI - "OZONE IN BIOLOGIS TA MEDICINI" - 2005 r., N.Novgorod"I Conference of Ozone Therapy of the Asian-European Union of Ozone Therapists and Medical Health" - 2006 r., Velikie Boldino, Nizhny Novgorod region VII - "OZONE IN BIOLOGIS TA MEDICINI" - 2007 r., N.Novgorod U111 – "Ozone, active formulation and methods of intensive therapy in medicine" - 2009 N. Novgorod Until 2000, the Russian School of Ozone Therapy had formulated its own form, the type of the European one, until the establishment of ozone as a preoccupation. The main reason is the wider improvement of the physiological dose in the ozone dose, the reduction of significantly lower concentrations and doses of ozone, the fragmentation of the technology of extracorporeal processing of the great blood oxygen concentrations (ozone recovery) In the case of the greatest Russian doctors, the most effective ozone concentrations know that the basic principle of medicine is “not ours”. Safety and efficiency of Russian methods of ozone therapy bagatorazovogo primed and brought 100 percent to the development of the field of medicine. As a result of the basic fundamental clinical dosages by the Nizhny Novgorod scientists, “It has been established that there is an inevitable regularity in the formulation of adaptive mechanisms in the organisms of workers with systemic infusion of low therapeutic doses of ozone, which triggers an antioxidant Intensification of radical reactions, which, in turn, increased the activity of enzymatic and non-enzymatic lanoks of the antioxidant system to destroy "(Kontorshchikova K. N., Peretyagin S. P.) ). The robots of the vichiznykh knew the development of new technology and the aspect of ozone management with the following advantage:- Wide range in the quality of ozone-degraded physiological rose (0.9% NaCl solution)
- Stagnation of proportionally small concentrations and doses of ozone during systemic infusion (intravascular and intraintestinal administration)
- Internal infusion of ozone
- Internal coronary introduction of ozonation is not cardioplegic
- Total extracorporeal treatment with ozone of great blood supply in case of piece blood circulation
- Low-flow ozone-oxygen therapy
- Intraportal introduction of ozone
- Zasosuvannya ozone at the theater
- Supportive systemic ozone therapy with biochemical control methods
- Ozone therapy is a non-drug method of lyuvalny infusion, which allows to eliminate positive results in case of pathology of the genesis.
- The biological activity of parenterally administered ozone is manifested at the level of low concentrations and doses, which is supervised by clinically variable positive drug effects, which may clearly vary the dose of accumulation.
- To inform the Russian and European schools of ozone therapy about those, how to study ozone in the capacity of a doctor, will significantly improve the efficiency of drug therapy, allowing for the substitution of pharmaceuticals in pharmacology. At the same time, ozone therapy introduces powerful acid reactions and processes of ailing organisms.
- The technical capabilities of modern medical ozonators, with the power of ultra-precise dosage, allow ozone to be consumed in the range of low therapeutic concentrations similar to those used in pharmacological conditions.
7. Where to lay the necessary hour of water treatment?
ozone health stay at the water and lie at the temperature
water and areas of gas contact with water.
Chim colder waterі less size rosіkach,
less ozone will be calculated. Chim vishche the temperature of the water,
Tim shvidshe ozone drops to sourness and is consumed with viparovanny.
Falling from the step wandering around
more and less ozone concentration is required.
8. What is necessary for additional filtration
drive pislya ozonuvannya?
Yakshcho water is mystila great number
complex processes, then as a result of processing
ozone in nіy vipadaut rіznі fall off.
This kind of water is needed additional filtering.
For a whole lot of filtering, you can choose the simplest and
cheap filters.
With a tsom resourceїх robots there will be significant progress.
9. Chi slid be afraid of a tricky hour
treatment with ozone?
Vodi processing overworldly quantity of ozone
do not pull the teeth of the inheritance.
Gas quickly turns into kissen,
I will only paint the brightness of the water.
10. A clear indicator of the acidity of water,
passed ozonuvannya?
water maє weak reaction PH = 7.5 - 9.0.
11. On skilshuua vmest
sour in the water of ozonuvannya?
vmist kisnyu in the water it will be 14 - 15 times.
12. How shvidko ozone fall off in the water?
In every hour after 10 min. ozone concentration decreases
by half, fixing water and water.
By the cold water after 15-20 minutes. ozone fall
half, pretending hydroxyl group and water.
13. Why should we drink sour water?
pidsilyu living glucose by tissues and organs
zbіlshu nasichuvanostі acidic blood plasma
change step sour hunger
paint microcirculation of blood.
nada positive diyu
On liver metabolism and nirok.
to the robot heart meat.
change the frequency dikhannya i
Zbilshu dichny obsyag.
14. How much do you need ozonuvat water?
chim bolsh weanim houses water,
Tim is more than an hour of processing.
So, for example, ozonation of 3 liters of water supply
loan 10 - 15 min.
Take the same ride taken from the waters,
in fallowness during the season, rock and rural areas
guilty is carried out in three-chotiri times more.
15. Who has more beauty to ozonuvat water in misci or a jar?
The dishes are more beautiful than the viberati I will make a sound
throat (jar) for increasing concentration
ozone in the interconnected society.
16. If you dye water for tea more beautifully,
until the end of the day?
For brewing tea water is not recommended
bring to the point.
Spinning t = 85-90 ° С.
Carry out the treatment of water before heating.
17. Is it possible to ozonize mineral water?
Such waters are available all minerals,
Wonderful and weathered sour.
Chi has honored you with a statty?
The interaction between ozone and ozone is responsible for the direct reaction with ozone molecules, or even with radicals, which are in the process of a drop. More active ozone in conjunction with anions, lower with neutral and cationic words.
Ozone, being an active oxidizing agent, interacts with both organic and inorganic words. With halogens with ozone does not react fluorine and practically does not enter into the interaction of chlorine. Bromine is oxidized with ozone from a mixture to hypobromite, and then to bromate z'єdnan. At the same time, bromide can be established in parallel with the interaction of organic activity with amiak. Iodine is oxidized with ozone even more high-grade and becomes iodate and iodic acid. Salts of halogen-containing acids are not susceptible to ozone oxidation. Practically neutral to ozone є nitrogen and half, including ammonia and ammonia, as well as nitrate itself, with the blame of aminiv, kindly interconnect with hydroxyl radicals. Toxic cyanides are easily oxidized by ozone in a cyanide, further oxidation of which grows even more often and accelerate only in the presence of ions of medium, to cope with the presence of ions in the valley. Water and sulphates are oxidized to sulphates when combined with ozone. Well, if there are reactions with metals, then ozone is actively oxidized by oxidizing zalizo and manganese, cobalt and nickel, peroxides and hydroxides, which can be seen at a break in flocculation or filtration. Chromium is practically passive to ozone;
»Article Ozone for water treatment... We'll talk about storing gas for more clean water.
Ozone for water purification is a technology that has been changed for an hour. Bilsh nіzh part of the European land vikoristovuyut ozonuvannya as a shorter method of purification of water. France became the first land, as the ozone was consumed with purified water.
The main reason for the ozone as a reagent in water treatment is due to the fact that it is not necessary to buy new elements, reagents, etc.
Ozone is an active chemistry with a combination of three atoms. It’s true that the third atom is easy to come up with and actively interact with your desires. On the whole, the technology of ozonation of water is based.
Ozone for the development of its advanced reactionary health oxidizing organic houses, robbing them with unreliable, enlarging them, with such a rank, increasing the efficiency of the offensive steps of purifying water, deceasingly
Ozone oxidizes in water, zinc, manganese, important metal, translate it in a non-critical mill and lie farther away.
The presence of unacceptable and loud odors. As soon as the water is present with water and amiak, then the ozonation of water will increase the release of these words.
Ozone nadaє chastkovo antinakіpederavnoe dіyu. Ozonation of water and confidence in the establishment of calcium salts on the walls of a hot pipeline and often saw a cold deposit.
The modern technologies of ozonation of the staff of the provincial staff make everything less and less expensive. Oskilki effect ozonuvannya complex, then with purified water for the whole booths near bagatokh vipadkah, especially with "important" water, you can transfer the included technology.
Butt of organizing water purification for additional ozone.
It’s not a recipe for use, but it’s just shown on the butt, as it can be used for ozonation in water preparation.
Supposedly, the situation: the clear water should be replaced by 2.5 mg / l of the broken saline, the oxidation rate is 12 mgO2 / l, the calamity is 5 mg / l, the color is 30 degrees. Tobto, the water is calamut, green, rich in organics and hair. Chi is not a good situation, a simple iron remover can fit in with it. Ale, admittedly, mi zbiraєmosya zastosuvati mensh vitratne ozonuvannya.
The basic rule of thumb is that the dose of ozone for the treatment of water should be 0.14 * when the amount of water is visible, so that 0.14 is multiplied by the concentration of water. Dzherelo I'm sorry I don’t remember. Our ozone dose is 0.35 mg / l. Oscillation of oxidation is a complex indicator, and as a matter of fact, it is not possible to be there, then it is precisely possible to deplete the dose of ozone only in practice. Ozone in our application requires 2 mg / l. Apparently, for 1000 liters, 2000 milligrams of ozone is required, or 2 grams. 1000 liters - a lot of water, as you need 3 3-4 people to add.
Ozonators vary by productivity: 1 g / year, 2 g / year, 4 g / year, etc. Chim more grams for a year, Tim dear. Admittedly, the ozonizer was vibrated by 1 g / year. This means that it takes 2 years to get the drive behind our butt. Yak will we supply ozone? It’s even simpler - to gurgle with a compressor in the accumulating bang. Bulbashki, filled with ozone, pass through the water, oxidize everything that can be oxidized, and burst on the surface of the water. Not vikoristany ozone needs to be seen, so as ozone to finish off the bran. For the whole on the outputs from the tank, a filter will be installed with active components, such as an ozone storage facility. Everything is worthy of being well-ventilated.
The water rises, the bulk and the organics grow larger, and it is already possible to be filtered at the advanced stage of water purification, and behind the additional extraordinary filters of mechanical purification of the cartridge type. We will not take a look at the filter from the active filter and the part of the industrial filter. Ale tse still needs to be amazed for pennies.
Also required: an ozonator with a productivity of 1 g / year, a storage tank for 1000 liters, a compressor for supplying ozone-nutritive sums to the tank, a system for supplying ozone to the tank, a coarse filter, a pump at the station, filters for water purification of the mechanics.
Schematically, it will look like this:

Otzhe, the water rises from the upper reaches, to collect in the Umnist. Water level and regulating the float to the pump and solenoid valve. All at once connect to the timer, which allows you to dial in the driver only at night. The second timer includes an ozonator and a compressor for feeding the ozone-ozone sum into the water. Programming timer for 2 years of robots. After 2 years, the ozonator and compressor are switched on.
For 2 years, ozone is consumed in the tank through a hose with dowels for equal supply of ozone throughout the entire volume of the tank. Zalizo oxidize, organics oxidize, stench grow larger and fall into siege.
When the baggage stand up, turn on the tap - and the pumping station also supplies purified water through a number of filters (for example, 100 microns, cartridge corrugations for 30 microns, cartridges for 5 microns and filters)
As a result, the water doesn’t take revenge on it;
In order for us to see the houses we will increase, it will simply take an hour of ozonation. The procedure for the experiment of simplicity - they poured water into the tank, missed ozone for 2 years, a year, 3 years, 4 years, and adjusted the appearance of the water.
It is necessary to remember that ozone may become more and more unbaked in the water, and it will become safe for people for 20, and for vernosity - for 30 chilin. Tobto, you can drink water only in an hour.
Vazhaєmo hour: the ear of the tank about the first year of the night. Replenishment of the tank 2 years - 3 years. An hour for the destruction of ozone in water - 30 quilins. 3.30 nights - the water is ready before the weekend.
Vitrati for the project of minimals, from the small elements - only cartridges of mechanical cleaning in the middle filtration, like boules in the presence of any water treatment scheme - with ozone, and without ozone. The other winter elements and vitrate materials are not used - they do not replace the catalytic preservation, nor the vitrate for manganese or strong.
De take ozonatori? Basically, quiet companies that are engaged in swimming pools. Stink and show and show, and, you can and stand.
Thus, ozonuvannya with the right approach - tse complex water purification.

For materials http://voda.blox.ua/2008/10/Kak-vybrat-filtr-dlya-vody-34.html
With normal sinks, ozone is gas-like, barren-free speech, with a volodya pungent smell. Vvazhaєtsya, the smell of ozone is the smell of fresh food, and a threat. It’s worthwhile so, if only in that case, if the concentration is even lower and becomes a fraction of the boundary permissible concentration (HDC). A detailed description of the physical and chemical powers of ozone can be seen in numerical robots, zokrem. deyakі main physical and chemical authorities ozone guidance in table 1.1.
1.1. Basic physical and chemical power to ozone.
Ozone tolerance in water
When ozone is determined in water, its concentration gradually increases and reaches the boundary values for these conditions. (Voz / Vv), or in absolute values for ozone (g / l). At the same time, it is important that the process of deciding is to comply with the law of Henry, but the amount of ozone is proportional to the grip of gas-like ozone over the solution.
Cstat = β M Pγ, g / l
De: Cstats - ozone resistance, g / l; β - Bunsen coefficient; M - ozone concentration = 2.14 g / l; Pγ is a partial grip of ozone in the open gas environment.
It means that ozone is responsible for the main atmospheric gases - nitrogen and acidity, a little less oxidizing agents such as chlorine and chlorine dioxide. The dissolution of ozone quickly changes with lower water temperatures.
Distribution of ozone in water
One hour, the ozone is delivered to the water. With a wide swiftness of the first drop, as the zoomed value of the "hour of life", it is possible to accumulate in the water temperature and, in the main, from the water storage in the first place, because of the manifestation of the water of the small houses, especially of the organic metal springs. The position of the good to be illustrated by the data, aiming at the little one 1.2. 
Malunok 1.2. ... Distribution of ozone in other types of water at temperatures of 20 ° C.
- Double-distilled water. 2. Distillate. 3. Water "Z-pid tap". 4. The water of the Tsurikhovskoye lake was filtered.
An hour of life in one-time distilled water is 20 hilines, and in extraordinary water there is a sprinkle of hilin.
Reaction to ozone with inorganic words.
Ozone can be reacted with growth, which is found in the water by two different mechanisms: without the middle as ozone (in molecular form) and in the presence of the OH * radical, like a wine when ozone falls in water. Vvazhaєtsya, scho in neutral water 2 channels of reactions rozpodіlenі occasionally. In the acidic middle, the re-fermentation has a molecular mechanism, and in the lazy one, it is radical. Ozone particles are present in chemical reactions as oxidizing, then one can judge about this oxidative health by, so called, the value of the oxide potential. The values of the values of the oxidation potentials of the small rivers, as well as the oxidation potentials, are shown in Table 1.3.
oxidizing |
Oxidation potential (Volt) |
Introduce. Oksli. potential up to OK pit. chlorine |
hydroxyl radical |
||
atomic acid |
||
Peroxide |
||
chloronuvatic acid |
||
chlorine dioxide |
||
Table 1.3. viply, and ozone is the strongest oxidizing agent. For stable talk, only fluorine is used, the reaction rate to ozone can be assessed by cob fluorine, which is the exact value of the reaction to ozone. Chastkovo tsі danі presented to the baby 1.3.
Reaction to ozone with metals
They are easily oxidized by ozone and manganese hydroxide and manganese dioxide. Permanganate can also make statements for such a reaction: 2Mn + ² + 5O3 + 3H2O □ 2MnO4ˉ + 3O2 + 6H + In parallel with the cym process, the process is carried out - the depletion of manganese from the range:
2Mn + ² + 2O3 + 4H2O □ 2MnO (OH) 2 ↓ + 2O2 + 4H + Oxidation of ions in manganese oxide, cobalt and nickel is used in liquids, as the values of liquidity constants 1 / liquid are given by the order of magnitude. Chromium can be oxidized to hexavalent chromium. The process of folding, vimag of special minds. 5 6 Lead is oxidized with PbO2 ozone with a reaction rate constant of close to 10 -10 l / mol sec. Metals that make complexes with EDTA, such as Pb, Ni, Cd and Mn, sometimes go through the stage of ruining the complex, and then oxidize. Such reactions are produced by the complexation of metals with natural humic acids.
Reaction to ozone organic spoluks.
The electronic structure of the bipolar ozone: from one side - negative, from the other - positive. For the reason, ozone can react at once as electrophilic as well as nucleophilically. Typically, in the reaction of direct oxidation of rivers with ozone in water, the electrophilic mechanism is used. Rozumovskiy. It is very important to give a characteristic of the reaction of all the main organic phrases with ozone. Looking directly into the ozone, it is possible headquarters: Nasic alcoholic compounds react with ozone even more frequently. Most chlorinated in carbohydrates and navit, non-saturated in carbohydrates do not react without ozone. In general, it is necessary to mediate the interaction with ozone through the OH radical. Benzene is oxidized by ozone more often, and polycyclic in carbohydrates more.
The hour of reaction to ozone with phenolic halves becomes several seconds. The chastkovo reaction scheme for phenol is presented on little 1.5. Ion phenate reacts more quickly, less protonated phenol. It means that the constant speed is even greater and close to the phenols of Riznoi Budovia. Carboxylic acids, ketic acids and a number of similar products are stable products to the process of oxidation of organic fluids by ozone. Amini at neutral pH values react with ozone, at pH> 8, oxidation reactions take place more quickly. However, in the main, the reaction of oxidation of aminiv goes through OH radicals. Quarter aminis (aromatic aminis) react with ozone shvidshe. Alcohol can interact with ozone, making it possible for intermediate spoluk hydroperoxide. With a whole stench, it is oxidized to carboxylic acids, at that hour, like second alcohols, to ketones. Carbonic acids react weakly with ozone or do not react with ozone.
Mercaptan is oxidized with ozone to sulfonic acids. Bisulfite and sulfonates Amino acids, before the warehouse, which include sirka (cysteine, cestin and methionin), react quickly. Amino acids (warehouse part of bilkiv) react by electrical mechanism. Among pesticides, to take revenge of phosphoric acid ester, we are most likely to use parathion. Ozonuvannya tsyogo z'єdnannya to produce before the appearance of paraoxon, which is more toxic, lower parathion. Further ozonation re-transforms paraoxon into less toxic speech (for example, into nitrophenol, which is then oxidized to end products - nitrate and CO2).
When drinking water is sampled, the mechanism of direct oxidation through ozone in molecular form is basic. The constants of a great number of organics with ozone are presented in the Hoigne roundup.
- Ozone, as a microflori activator.
As soon as the food has gone bad, the ozone will force the bactericidal and virulent (anti-virus) action. good assessments The efficiency of the young disinfectants is well illustrated by the data of the little boy 2.1.

Malunok 2.1. The speed of decontamination of the pathogenic E-coli with different deactivating agents.
In the given hour, when evaluating the effectiveness of that one disinfectant, it is called the Z x T criterion, so that the concentration of the reagent is adjusted for an hour. You can say that DIA (Inactivation) = Concentration Hour of exposure. Table 2.1. presented for the appropriate value of SHT criteria for small microorganisms - disinfecting agents. Table 2.1. CGT criterion for small microorganisms (99% inactivation at 5-25 ° C. CGT criterion (Mb / L xv))
microorganism view |
Ozone pH: 6/7 |
Vilny chlorine pH: 6/7 |
Chloramine pH: 8/9 |
Chlorine Dioxide pH: 6/7 |
poliovirus |
||||
rotavirusi |
||||
Gardialyamblasti |
||||
Gardia myurism |
||||
Cryptosporidium |
||||
For 90% inactivation (1 log) |
Obviously, ozone overrides such disinfectants, such as chlorine, chloramine and chlorine dioxides. For such a pathogenic, such as intestinal stick (E-coli), ozone is more effective, less chlorine, a little more. At the same hour, for the cryptosporidium, the Criteria for the disinfectants approach up to 1000. To transfer, in principle, ozone can compete with such disinfecting reagents, such as chlorine, bromine, iodine, hydrochloric acid. , vyroblyayu chlorous acid HOCl, yak, in its own accord, dissociation in water for anion CEO and cation N. It was established that at pH = 8 the concentration of undissolved acid is ≈ 20%, and at pH = 7, the concentration of HClO is ≈80%. So, since it is a strong bactericidal effect of low HCLO, in case of vicorian chlorine (in the presence of hypochlorite), it is necessary to reduce the optimal value of pH.Iod, as a disinfectant, in the case of vicorist flotation systems, it is not necessary to provide water for the deterioration of water. Due to its disinfecting power, iodine is weaker than chlorine and more than ozone, but it is more efficient in transportation. low value of GDK. The problem is the approval of bromates in the case of ozonated bromine, to replace the water with a serious dose, in the distribution "Victorian ozone for the preparation of drinking water." However, all the stench may seem like some shortcomings and a great extension until the present hour. ” In the Danish hour, chlorine will be washed in special apparatus-Chlorate, when electrolyzing the kitchen salt, or hypochlorite, which can be found in water, requires a concentration of free chlorine. It is necessary, by and large, for the term "strong chlorine" to increase the concentration of chlorinuvatist acid and HClO. Chlorinators victoriously requires a reagent reserve, and hypochlorite, when it develops, drops out and drops in chlorine. - Chlorine, vaping gas (GDK for chlorine is 1 mg / m³). Win vpershe buv vikoristaniy yak boyova otruyna zasib in persha svitovu vіynu There are many victims of this bully. Ozone can be classified as toxic gases and the HDC can reach a low level (0.1 mg / m³). Happily, ozone has a super-strong characteristic odor, and people perceive the presence of ozone in some of the earlier, lower concentration of a still unsafe value (scent time ≈ 0.1 / 0.5 GDK). It is necessary to be cared for, before the present hour of an unprecedentedly deadly one, to see an important one, which is responsible for the state of health of the state, in the form of ozone depletion. The nutritional toxicity of ozone will not be considered separately. One of the main inappropriate powers of chlorine is that, in the case of its reaction with a large number of organic compounds, the spectrum of organochlorine children, a large number of chlorophylls and poisons are highly toxic. From the strongest species in Denmark, there will be some organic breaches, and moreover, there will be a lot of toxins in the population in the ruined immune system. So, talking about dioxins, inodi vikoristovuyt term "chemistry SNID." Quite a speech can be done with a weak disinfectant, and a little superficially strongly tease the mucous membranes of the eyes and the nasopharynx. Chloramini is often called "tied with chlorine." The range of dressings chlorine is 5-10 times more powerful, less chlorine. Ozone can also be used for industrial use (by products) for ozonation of gas and condensed mixtures. Theoretically, it is possible to admit that it is accepted by products more toxic, less ozone. This problem has been the subject of many years ago. Concentration and warehouse of industrial rivers, which are detected when ozonized, even lie down because of the fact that the water is ozone. Crazy, in the first place to pretend to be less by products і the warehouse їх more obvious. All food will be looked at from the general distribution around. It is possible to summarize the amount of food for those who are very rich in advance of the rank:
- The overwhelming majority of industrial products, oxidized by ozone, are less toxic, less toxic.
- Directly correlating intermediate speeches, which is done during the experimental experiments with chlorination and ozonation, has shown that in the first place it is possible to pretend to be more by products.
2.1. Chlorine and ozone disinfection at industrial cleaning stations and in laboratory minds.
Bagatorial history of registration of two disinfectants at great water treatment plants to avenge a wealth of factual material, so that one can judge about their crossings and shortcomings. A number of many examples are generated near the bottom of the "Ozonuvannya Vody"
At the Belmont filter station in Philadelphia (USA), ozonation of water gave more successful results in terms of e-coli, lower results achieved with chlorination. The effect of water contamination with ozone in the case of extravagant quantities of bacteria in 1 ml 800-1200 od. to become: at a dose of 1 ml / l ozone - 60-65%, at a dose of 2 ml / l - 85%, at a dose of 3 ml / l - 90-95%. An acceptable dose of ozone was added to 3-4 ml / L at the Rublevskaya water supply station (Moscow metro station) ozonation of the waters of the Moskva rivers was carried out. The quantity of bacteria in 1 ml of water was reduced by 92-99% in the intervals between the hours of 1-25 minutes. The bactericidal dose to ozone was given such, for the filler, which cannot be e-coli in 500 ml. drive. Adjustment of calamity from 6.8 to 12 mg / l and coloration from 3.2 to 18 Greden. Inimal to increase the bactericidal dose of ozone from 3.2 to 4.1 mg / L. The "Rydenor" and "Ingols" stations from the USA were doused with chlorine and ozone e-coli suspensions in distilled water at Hp = 6.8 and at a temperature of 1 ° C. , so that 99% of e-coli colonies die down, they became 0.25-0.3 mg / L for 16 minutes for chlorine, and 0.5 mg / L for 1 minute for ozone. Chart 2.3. it can be seen that the curve of bacteriological decline is due to the increased dosage of chlorine, and, moreover, there is an approximately exponential decline in the number of bacteria. In case of ozonation, a picture is observed - with low concentrations of ozone, the amount of ozone in the bacterium is insignificant, but it is corrected from a very critical dose (0.3-0.5 mg / l) ozone increases significantly and more chlorine is added to the microorganism, in some part. Ozone is required for bactericidal activity. singing hour... At the same time, the whole mass of bacterial species is ozone. Chlorine viroblyaє vibіrkovе otrunnya zhitєvyh centersіv bacterіy, moreover, it is necessary to add more after the need for a trivial hour for diffusion in protoplasm.
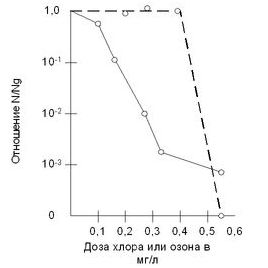
The graph of the survival of bacteria during the treatment of water with ozone and chlorine - - - - ozone -------- chlorine
2.4. Diya ozone on super-streams, cysts and other pathogens.
According to the microflora, ozone is also an effective bacterial agent. According to Bringmann, Bacillus subtilis was prone to ozone in water 3000 times more, less chlorine. M. Kane and Glöckner injected ozone and chlorine on cysts (thin membranes, which are set up on about one-line organisms) Endamoeba hystolica on bacteria, subtypes of cultures. Installed, about an hour, it is necessary for the depletion of cich organisms with an excess concentration of ozone of 0.3 mg / l, a warehouse of 2-7.5 minutes, and for chlorine (an excess concentration of 0.5-1 mg / l) for more chlorine - 15-20 minutes.
Diya ozone on virus
Ozone nadaє yaskravo virazhene, a radical infusion on a lot of viruses, which supervises more and more fluff of viral protein. In the 1940s-60s, US virologists and Nimechchines conducted a number of additional tests with suspensions of polyeemilite virus with the addition of additional chlorine, ozone and chlorine dioxide. Drawings from tsikh toddzhen can be presented in an offensive view:
- Inactivation of polioemilita virus with chlorine can be reached at a dose of 0.1 mg / l at a water temperature of 18 ° C, at a water temperature of 7 ºC, the chlorine dose is not less than 0.25 mg / l.
- Inactivation of the virus for additional ozone is reached with a dose of 0.1 mg / l at a water temperature of 18 ° C, for cold water -7 ° C, the dose is due to but increased to 0.15 mg / l.
- In case of vicorian dykoxide, chlorine needs a dose of 0.6 mg / l (18 ºС). For water with a temperature of 7 ºС, the dose of chlorine dioxide is responsible for 1 mg / l.
According to Naumann's danny, the polyeemilite zenizyutsya ozone for 2 minutes at a concentration of 0.45 mg / l, that is, for an hour chlorination with a dose of 1 mg / l for 3 years.
Ozone and hydrobionti
On the thought of a number of authors, ozone has successfully assimilated micro-hydro-growth and simpler, more active, less chlorine. So ozone at a concentration of 15 mg / l for 3 minutes is the most simple, as it retains its activity when sampled with a dose of chlorine of 250 mg / l for a trivial hour. At a dose of 0.9-1.0 mg / l of ozone, the larvae of the mollusk dreisenia lost 90%, at a dose of 2 mg / l - 98%, at a dose of 3 mg / l - increased. Longer forms of the molluscs were lost when they were more trivial with ozonated water (up to 30 min). True, a lot of water, you want to grow wildly near the very critical basins on sleepy lights, weak to the point of ozone. Here vikoristovuyut shock doses of chlorine. To carry out the cleaning at night during the preventive cleaning of such pools. By supplying the bags to the extremely short porous ozone, chlorine and chlorine dioxide, as an agent for the purification and disinfection of water, obviously Dіysnі, tо see the robots of water treatment plants, wіkо thе vicorіtіtіvіyu tо ozone аnd chlorine, bіnіtіnu lead to cinnamon ozone. Thus, V. F. Kozhinov, who set up a French water preparation station in Saint-Maure and a station in Chicago (USA), means that the first fall of ailments of "water travel" has been registered in 1 fall per 100 thousand. Residents who want the concentration of excess ozone in water did not exceed 0.05 mg / l. At the same hour in Chicago, there was a small town of slunk-intestine sickness in Chicago, which was not affected by chlorine at the water supply. ) taku thought: "Naybіlsh іstotnyh zaprechennya oppo ozonuvannya zvvazyut vіvazhayut vіdnіtnі ozone surplus ozone in the distributing water supply net, so as for an hour of chlorination in the amount of accumulation of surplus." Experiments carried out in Ashton (England) have shown that in the disinfected with ozone water, which circulates in the reference water supply pipelines, there is no deterioration of the quality. Control samples of ozone water, taken from the hedge, turned out to be absolutely equal to the samples, which were taken from the dzherel, so that the excess chlorine could be removed in the water. ... That is, the presence of excess chlorine in the pipelines does not mean the same bacterial purity of the water. One of the authors discussed the problem with the leaders of the Tsurikhovsky water supply system and approved M.T.B. disinfectants of the transfer of ozone are indisputable. Because of the strength, I will not look at the ozone. Nareshti, the problem of partost. I think that ozonuvannya is much dearer than chloruvannya. However, it is not so. In the process of chlorination of the wine, it is necessary to put the chlorine in water, to carry out the so-called dechlorination. To remove the water and the good quality, work is done, and special reagents are used. Due to the deterioration of this factor, as well as the tendency of an uninterrupted decrease in the price of ozone supply and the increase in the price of chlorine and chlorine products, in this hour the number of these processes is comparable. To what? Є number of reasons: - psychological reason. Pratsyuvati with chlorine, especially when it comes to baloni with rіdky chlorine, it's quite simple. To deliver the vents of the balloon or the capacity to the pool in the form of hypochlorite, as all the problems with the disinfection of the virus. Price, crazy, simpler, no quest for the concentration of ozone, how to go from the ozonator, but the ozonator is loosely folded, and we need to change it, but it is not possible to switch off. broadening to ozone. Until the very end of the hour, the need for ozonator possession was too much for him, and low rіven avtomatizatsії pripuskayut neobhіdnіst vikoristannya obslugovuyuchogo staff schodo visokoї kvalіfіkatsіі.Suschestvenny breakthrough in problemі stvorennya nadіynogo, clumsy in vikoristannі ozonation i ozonoіzmerіtelnogo obladnannya was mozhlivim pіslya appeared Suchasnyj IGBT tranzistorіv scho have allowed rіzko sprostiti i zdesheviti virobnitstvo іmpulsnih visokovoltnih generatorіv, rozvitok mіkroprotsesornoї tehnіki i novih tipіv ultrafіoletovih sensors, special synthetic zeolites and in. All the prices, as well as the results of the delay of pulsed electric discharges in every day, allowed the development of technological solutions We have introduced new possibilities for the production of ozonators in undried food, acid ozonators, systems for monitoring excess ozone in water, an ozonometer, kisnyu concentrator That is the best possession, how to prevent ozonuvannya zonuvannya nagato more forgive and handy technology, but not before. Our radios, our last generation and distribution, seized by patents in Russia, the USA, Japan and other countries, will help the life of people of greater simplicity, without blessing, and, let us help, do it.
List of Literature
Draginsky V.L., Aleksova L.P., Samoilovich V.G. "Ozonuvannya in the processes of purification of water" M. Deli print. 2007 r
Інж. V.V. Karaffa-Korbutt "Ozone 'and yogo zasosuvannya In industry and sanitarii" Ed. "Osvita" SPP. 1912 RUR
V.F. Kozhinov, I.V. Kozhinov "Ozonuvannya Vodi" M. Stroyizdat 1973 r
V.V. Lukin, M.P. Popovich, S.N. Tkachenko "Physical chemistry to ozone" Ed. MSU 1998r.
Manley T.S., Negowski S.J. Ozone in Encyclopedic of Chemical Technology. Second Ed. Vol 14. N.J. +1967.
Hozvath M. H., Bilitrki, haud., Huttez. "Ozone" Ed. Akademie. Kiado. Budapest one thousand nine hundred sixty seven
B.F. Kogan i br. "Dovidnik on razchinnosti" v.1, book. 1, M. thousand nine hundred sixty one.
Masschelein W.J. "Processes unitaixes du treatmeut de l esu potable" Ed. CEBEIOC. Hiege. 1996.
Jore M., hegube B.J. Er. Hydrol. 11/14/1983.
Cowen W. Fetal. "Chemistry in water reuse". Ed. Ann. Azboz. Science Publ. Michigan. 1985.
Curol M.D. Env. Prog. 4.46.1985.
Hoigne J. "In Progress Technologies for water treatment" Ed. Plenum. Press # 3. +1988
Rozumovskiy S.D. і Zaika G.Z. "Ozone and yogo reactions with organic spoluks" M. 1974.
Hubner R. Gesundheitstechnik No. 12. 1 973.
Dojbido J. Etol. "Osvita of industrial rivers in the process of ozonation and chlorination" Wat. Res. 1999. 33. No. 4 р3111 - 3118.
Ridenour G.M., Inglols R.S. American Jounal of Public Health 1946. 3.6p 639.
Gomella C. 2e treitment d eux par l ozone. Extract du mensuel du ceutre Belge 287.1,967.
Kozhinov V.F. Ozonuvannya vodi. "Miske Gospodarstvo Moskvy" 1970. No. 7.
Steinhardt. Stadtehygiene. 1S. 1956.
Naumman E. "Das gas nnd Wassertach" 1952. NY.p.81.
Dickerman J.M. etral. Journ of New England Water Works Ass. 11/1/1954.
Shalashova E.S. "Appointment to ozone for purification of water by the living of the state" №6. 1960.
Thorp C. E. Jnd Med and Surg. 1950. v19 p 49
M.U. 2.1.2.694-98. "Vikoristannya ultraviolet vimіrі in case of infected waters of swimming pools".
G.I. Rogozhkin. "Purification and infection of water in the pools" Santekhnika. 4.2003. side 4-9.
